
The valence electrons occupy higher levels due to the increasing quantum number (n). 122:5132 (2000).\( \newcommand\): Periodic Table showing Atomic Radius Trendĭ own a group, atomic radius increases.
#Arrange these elements according to electronegativity. how to#
Three references are required to cover the values quoted in the table. How to use the table of initial isolation and protective action distances. A: Cyclopentadienyl anion is a five membered cyclic compound with two conjugated double bonds and one. A: The term electronegativity for a given atom can be defined as a measure its ability to attract. Solved Arrange the following elements in order of increasing - Chegg. Q: Using only a periodic table as a guide, arrange the atoms in order of decreasing electronegativity. Pauling, The Chemical Bond, Cornell University Press, Ithaca, New York, 1967. According to the Paulings scale of electronegativity, these metals ions should be.
View solution > Arrange F, C, O, N in the decreasing order electronegativity. The electronegativity of the following elements increase in the order : Medium. Dean (ed), Lange's Handbook of Chemistry (15th Edition), McGraw-Hill, 1999 Section 4 Table 4.5, Electronegativities of the Elements. Click hereto get an answer to your question Arrange the following elements in the increasing order of electronegativity. C < N < O < F Electronegativity decreases as you move down a group in the. Pauling, L., The Nature of the Chemical Bond, Third Edition, Cornell University Press, Ithaca, New York, 1960. Electronegativity increases left to right across a row in the periodic table e.g.Boca Raton, Florida, 2003 Section 9, Molecular Structure and Spectroscopy Electronegativity Lide (ed), CRC Handbook of Chemistry and Physics, 84th Edition. Keiter in Inorganic Chemistry : Principles of Structure and Reactivity, 4th edition, HarperCollins, New York, USA, 1993.Īs quoted from these sources in an online version of: David R. 95 (22 ratings) Transcribed image text: Arrange these elements according to electronegativity Most electronegative Least electronegative K Cs O Ga N. This is especially problematic for francium, which by relativistic calculations can be shown to be less electronegative than caesium, but for which the only value (0.7) in the literature predates these calculations. Arrange the following as per the instruction given in the brackets: (i) He. Seeing chemical elements arranged in the modern periodic table is as familiar as seeing. (ii) Boron has the highest electronegativity because These questions are for. Many of the highly radioactive elements have values that must be predictions or extrapolations, but are unfortunately not marked as such. Look up chemical element names, symbols, atomic masses and other.The suggested values are all taken from WebElements as a consistent set. 4 4 So most electronegative atom 0 oxygen Least electronegative cs Because we knows that electronegativity is the tendency to attract shared paill of. Electronegativity is not a uniquely defined property and may depend on the definition.Separate values for each source are only given where one or more sources differ.

The Inaccessible Earth: An integrated view to its structure and composition. However, francium is expected and, to a small extent, observed to be more electronegative than caesium. The base value of hydrogen was later increased by 0.10 and caesium's electronegativity was later refined to 0.79 however, no refinements have been made for francium as no experiment has been conducted.

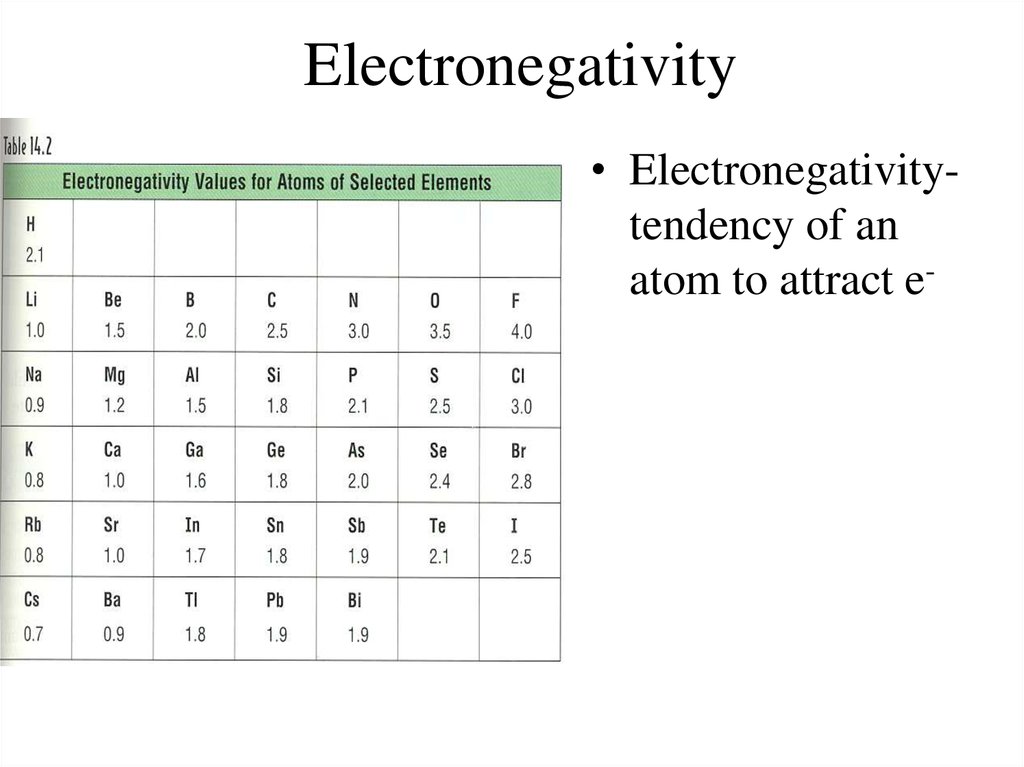
It can also be used to predict if the resulting molecule will be polar or nonpolar. Electronegativity is used to predict whether a bond between atoms will be ionic or covalent. Values for electronegativity run from 0 to 4. Electronegativity is defined as the ability of an atom to attract electrons towards itself. There are no reliable sources for Pm, Eu and Yb other than the range of 1.1–1.2 see Pauling, Linus (1960). Electronegativity is a chemical property which describes how well an atom can attract an electron to itself. See also: Electronegativities of the elements (data page) → Atomic radius decreases → Ionization energy increases → Electronegativity increases →


 0 kommentar(er)
0 kommentar(er)
